
The 4WARD Study is a phase 3 clinical research study that will soon be enrolling people in the U.S. living with chronic neutropenia. Researchers aim to learn if mavorixafor, the investigative study medicine, may increase neutrophil counts and decrease the chance of getting infections.

Chronic neutropenia encompasses a group of rare blood disorders that can be:
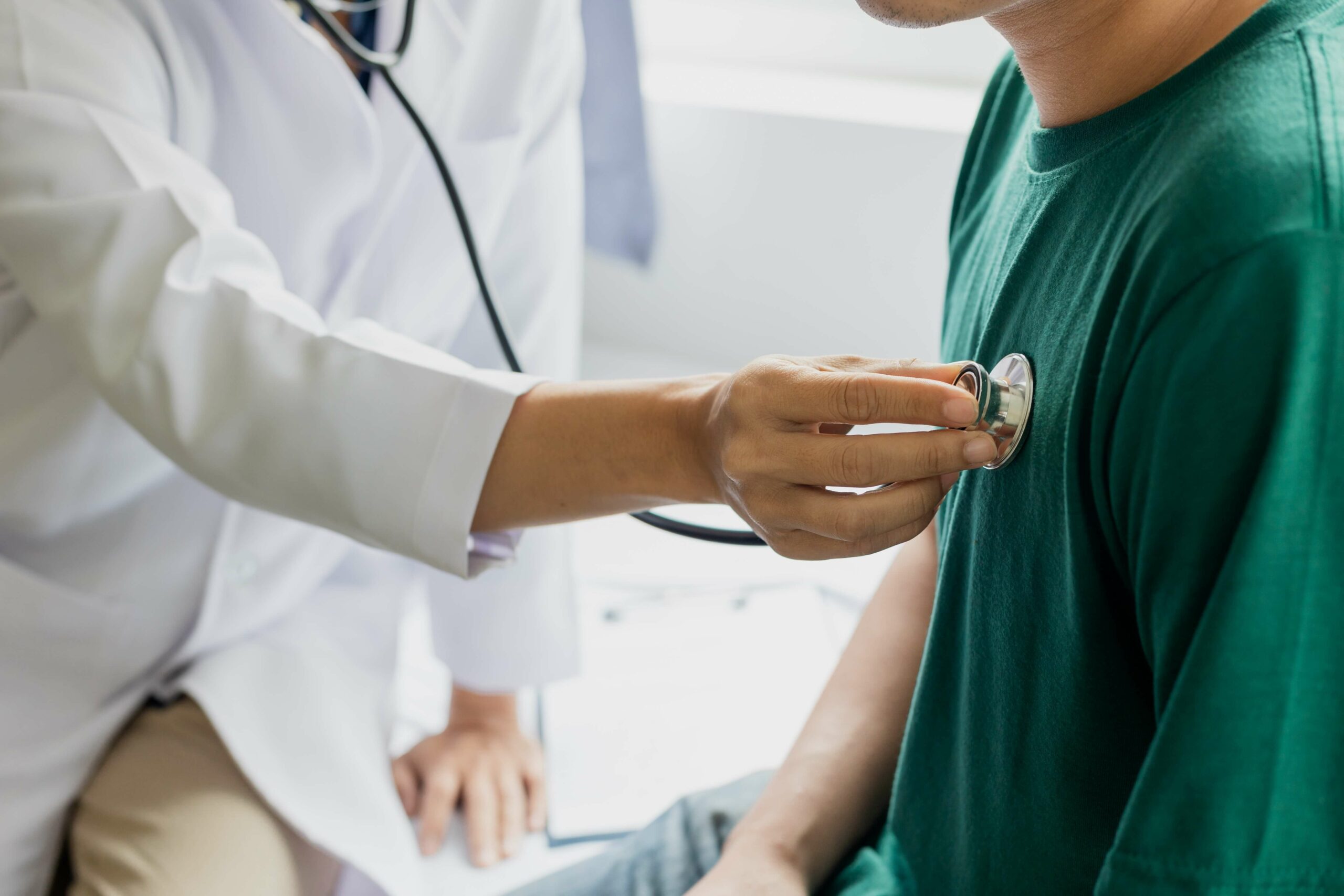
Neutrophils are a type of white blood cell that helps the body defend itself against infections. People with neutropenia have a low number of neutrophils in the blood. When neutrophil counts are low, there is a greater risk of infections such as ear infections, pneumonia, gingivitis, skin infections, and more.
Chronic neutropenia is diagnosed through a blood test. Genetic testing can be used to help identify or rule out congenital neutropenia.
Chronic neutropenia is a disease with few approved treatments, so more treatment options are needed. Current treatments include granulocyte colony-stimulating factor (G-CSF), an injection that helps the body make neutrophils, and intravenous immunoglobulin (IVIG), an infusion of antibodies to help your immune system fight off germs and disease.

You may be eligible to join the study if you:
For more details, visit https://clinicaltrials.gov/ct2/show/NCT06056297
Interested in joining? Please contact ClinicalTrialInfo@x4pharma.com.

Potential study participants will meet with the study team for a screening visit to determine if they meet the eligibility criteria and ensure that the risks and benefits are understood. This is called the informed consent process. Deciding to enroll in a study is a personal decision.
This study to learn about the investigational study medicine, mavorixafor, will last about 12 months.
Study participants will be randomly assigned to get either:
OR
Study-related treatments and procedures are provided at no cost to participants. If needed, approved travel, lodging, and meals will be reimbursed by the study sponsor, X4 Pharmaceuticals.

At study appointments, the study team measures the effects and the safety of the study medicine on your neutropenia by drawing blood and administering short questionnaires about your symptoms. Phase 3 study participants will undergo an ophthalmology (eye) exam and other exams and procedures.
The 4WARD Study is sponsored by X4 Pharmaceuticals, Inc., a pharmaceutical company developing treatments for diseases of the immune system, with a focus on rare diseases and those with limited treatment options.


Enter your email address to receive updates about this chronic neutropenia study.